Knowledge Hub

How Chronos® redefines HLD with FDA De Novo Clearance
Endocavitary ultrasound probes, used daily in gynecology, radiology, and emergency departments, are classified as semi-critical medical devices under the Spaulding classification. As such, they must undergo High-Level Disinfection (HLD) between each patient.
However, despite the use of protective sheaths, up to 13% of vaginal probes remain contaminated with HPV after manual reprocessing.1 This virus, responsible for 99% of cervical cancer cases2, is among the most resistant pathogens, alongside hepatitis B and C viruses.3&4
It is estimated that nearly 70% of Healthcare-Associated Infections (HAIs)5 could be prevented through improved hygiene practices and greater infection prevention efforts. High-Level Disinfection systems for medical devices are regulated by the FDA (Food and Drug Administration) as Class II medical devices. The FDA imposes strict validation criteria for any disinfection system: proven efficacy against abroad spectrum of pathogens, reproducibility of cycles, safety of use, and performance under real clinical conditions.
To meet these requirements, Germitec developed Chronos®, the first UV-C-based HLD system to meet FDA standards (De Novo clearance). This chemical-free technology redefines disinfection standards in clinical practice.
UV-C Disinfection with Chronos®
Developed by Germitec, Chronos® is a UV-C High-Level Disinfection system effective against a broad range of microorganisms.
This technology works through a physical mechanism of action, damaging the nucleic acids (DNA and RNA) of microorganisms — without the use of chemicals.
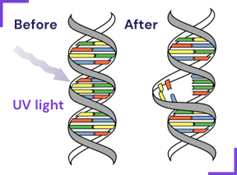
UV-C photons induce thymine base dimerization, thereby interrupting DNA replication.
The precisely controlled UV-C dose, delivered by three integrated photodiodes within the disinfection chamber, enables rapid inactivation of even the most resistant pathogens, such as Bacillus subtilis spores.
Regulatory and scientific validation meeting U.S. Standards
On August 28, 2024, Chronos® received FDA De Novo clearance, becoming the first UV-C disinfection device validated for both endocavitary and external ultrasound probes.
This recognition is based on a series of rigorous evaluations:
1. UV-C resistance hierarchy
- Internal testing and literature review6 established a microbial resistance hierarchy specific to UV-C, distinct from that seen with chemical disinfectants.
- This highlights the need to consider UV-C’s unique mechanisms when evaluating disinfection efficacy
2. Microbicidal potency testing
- An innovative methodology (Robot Droplet Inoculation of Carriers, RDIC) was developed to deposit numerous micro-droplets of pathogen suspension onto a5 cm² silicone test surface.
- A broad evaluation spectrum included vegetative bacteria, spores, mycobacteria, fungi, and viruses.
- Results: Full compliance with FDA High-Level Disinfection requirements, demonstrating sporicide, bactericide, fungicide, mycobactericide, and virucide efficacy during the HLD cycle
3. Simulated use testing
- A selection of endovaginal, transrectal, and external probes was chosen for their complex geometries.
- Multiple critical areas, including those most difficult for UV-C to reach, were inoculated with the most challenging microorganisms.
- Results: Demonstrated >6-logmicrobial reduction across all probe types, including hard-to-reach areas.
4. In-use testing
- Conducted across multiple healthcare centers to evaluate Chronos® under real clinical conditions and real microbial challenges.
- Included endovaginal, endorectal, and external probes used without sheaths and cleaned with a dry wipe prior to disinfection.
- Results: After one automated disinfection cycle with Chronos®, the average residual contamination level was <1.5 CFU/probe, below the detection threshold
Conclusion
With its automated, fast, and reproducible UV-C technology, Chronos® delivers a robust solution that meets the highest standards of High-Level Disinfection.
Fully compliant with FDA requirements, Chronos® sets a new benchmark in infection prevention, particularly in gynecology where effective probe disinfection is essential to protect both patients and healthcare professionals.
It is also compliant with and validated according to BS, TGA, and the European standard EN 14885:2022.
-----
References:
1. Leroy S. Infectious risk of endovaginal and transrectal ultrasonography: systematic review and meta-analysis. J Hosp Infect. 2013;83(2):99-106. doi:10.1016/j.jhin.2012.07.014
2. World Healthcare Organization 2023 - https://www.who.int/health-topics/cervical-cancer#tab=tab_
3. Meyers C, Milici J, Robison R. UVC radiation as an effective disinfectant method to inactivate human papillomaviruses. PloS One.2017;12(10):e0187377. doi:10.1371/journal.pone.0187377
4. Pichon M, Lebail-Carval K, Billaud G, Lina B, Gaucherand P, Mekki Y. Decontamination of Intravaginal Probes Infected by Human Papillomavirus (HPV) Using UV-C Decontamination System. J Clin Med.2019;8(11):1776. doi:10.3390/jcm8111776
5. Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32(2):101-114. doi:10.1086/657912
6. Masjoudi M, Mohseni M, Bolton JR. Sensitivity of Bacteria, Protozoa, Viruses, and Other Microorganisms to Ultraviolet Radiation. J Res Natl Inst Stand Technol. 2021;126:126021. doi:10.6028/jres.126.021





