Knowledge Hub

What criteria guide hygienists in choosing High-Level Disinfection (HLD) systems to prevent infection risks?
Key criteria
Disinfection of semi-critical invasive devices, such as ENT endoscopes or endocavitary ultrasound probes, is a crucial step in preventing the transmission of pathogens responsible for Healthcare-Associated Infections (HAIs).
To protect both patients and healthcare staff, primary objective is infection control and the choice of a disinfection system cannot be left to chance: it must rely on rigorous, well-defined criteria.
1. Regulatory compliance
Each year, 4.3 million cases of HAIs are reported in Europe1 and a significant proportion is linked to contamination from inadequately disinfected Medical Devices, such as endocavitary probes. These infections, caused by pathogens like Staphylococcus aureus, Enterococci, or HPV can lead to severe complications or even death.
To reduce the number of infections, disinfection systems are subject to strict regulatory requirements. For France, French Ministry of Health and SF2H worked side by side on explicit guidelines compiled in the "Best Practice Guide for the Reprocessing of Reusable Medical Devices (2022)".
Their recommendations are:
- For endocavitary probes, systematic Intermediate-Level Disinfection between each patient.
- For each examination, use of a single-use protective sheath.
According to Spaulding classification, reusable Medical Devices are classified as semi-critical as they may be in contact with non-intact skin, mucous membranes or blood.
Therefore, disinfection systems must be classified as Class IIb Medical Devices.
Why Class IIb?
Automated disinfection units are considered active Medical Devices, according to classification rules 9–13 of EU Regulation (MDR) 2017/745. Most importantly, Rule 16 (Annex VIII) applies to devices intended to disinfect invasive Medical Devices:
- Class IIa for non-invasive devices disinfection only.
- Class IIb is required for invasive devices such as endovaginal ultrasound probes or ENT endoscopes.
To claim High-Level Disinfection (HLD), manufacturers must also provide robust performance evidence.
2. Disinfection efficacy
Standard NF EN 14885:2022 evaluates the effectiveness of disinfection devices and chemical disinfectants. It defines the standards required for an effective microbial activity on bacteria, yeasts and fungi, viruses, mycobacteria and spores.
The table below summarizes the standards, the strains tested and the acceptance criterion for Chronos® Max.
Acceptance is based on logarithmic reduction after a complete HLD cycle.
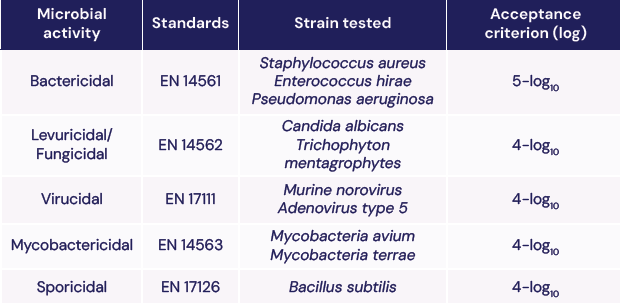
For example:
- Enterococcus hirae and Staphylococcus aureus, involved in Infectious Endocarditis, must be effectively eliminated.
3. Traceability
Traceability is a key element in infection risk management. It is essential to identify which device has been disinfected, by whom and when, in order to:
- Limit cross-contamination risk,
- React quickly in the event of an incident or suspected contamination chain.
SF2H guidelines (2022) emphasize that "traceability of disinfection procedures is mandatory": Medical Device and its disinfection must be documented for each patient.
French Ministry of Health goes further in Instruction DGOS/PF2/DGS/VSS1/2016/220 (July 2016), recommending cross-traceability between patient, probe (or Medical Device), and performed disinfection.
Manual traceability is accepted, but digital traceability is strongly recommended to ensure completeness, security and accessibility2.
4. User feedback
To make professionals’ daily lives easier and improve their Quality of Life at Work, while strengthening the safety of care, manufacturers must listen to user feedback. Collaborating with Healthcare Professionals, particularly End-users, is key to ensuring ease of use, workflow integration and adoption.
Trials allow to gather objective feedback and evaluate the:
- System’s ability to meet healthcare facility's need,
- Optimisation of the workflow: time saving, ease of use and human error risk reduction,
- User-friendly product and quick user training,
- Quality of Life at Work: reduction to chemical exposure, elimination of the risk of burns or toxic fumes.
End-user feedback help understand the practical needs and constraints to select a solution that is microbiologically effective AND adapts to daily healthcare facility use.
How does Chronos® Max meet all requirements?
Choosing the right disinfection solution is essential for Hygienists and Infection Preventionists.
Chronos® Max by Germitec stands out as a reliable, compliant, and high-performance solution for UV-C High-Level Disinfection of invasive Medical Devices, meeting both regulatory and operational requirements.
✅ Regulatory compliance
- CE-marked, Class IIb medical device, compliant with EU MDR 2017/745.
- Designed for HLD of TEE probes and ENT endoscopes without operating channel, ensuring patients and healthcare staff safety.
✅ Validated efficacy
Independent tests carried out by the independent Eurofins Biotech-Germande laboratory confirm compliance with standard NF EN 14885:2022, with proven effectiveness against:
- Enterococcus hirae and Staphylococcus aureus, common HAIs pathogens.
- HPV16 and 18, known for their high oncogenic potential.
✅ Integrated and reliable traceability
With Chronos® Max, traceability is integrated, continuous and controlled:
- Medical Device automatic identification inside the chamber (patented technology),
- Printed records via an integrated printer,
- Digital records via Germitrac intuitive interface, with traceability data of HLD cycles performed.
✅ User-centered solution
By eliminating the handling or exposure to chemicals for HLD, Chronos® Max ensures an effective, fast and safe disinfection:
- Low energy consumption: short and energy-efficient cycle,
- Automated system: time saving and human error risk reduction,
- Immediate availability after disinfection: no waiting time, no rinsing, no wiping.
Learn more about Chronos® Max, our UV-C High-Level Disinfection device for Cardiology and ENT departments.
--------
* Product available in Europe and Oceania, not available in the US
References:
1. Source: ECDC point prevalence survey (2016–2017)
2. Instruction n° DGOS/PF2/DGS/VSS1/2016/220 du 4 juillet 2016





